FORMULATION AND CHARACTERIZATION OF SUGAR FREE FAST DISSOLVING ANTIALLERGIC TABLET
Abstract
Author(s): Divya Pathak*, Md. Semimul Akhtar
Allergic disorders are most common worldwide. It also affects the quality of life. Due to these reasons, there is a requirement to develop rapid action producing formulation to treat these allergic conditions. Purpose- The Purpose of the study was Formulation and characterization of sugar free fast dissolving anti allergic tablet. Method- Formulation was done by using direct compression method with the aid of Super disintegrants addition. Twelve formulations were developed using three different super disintegrants in varying concentrations in such a way that total weight of the tablet remains the same. Results- Based on the results, formulation (PF-9) containing 2% Ac di sol and 5% Polyplasdone XL-10 was identified as ideal and best formulation among all formulations. In vitro release of optimized formulation (PF-9) was found to be 98.80% and drug release within 10 min and in vitro dispersion time was found to be 25sec. Conclusion- Levocetirizine Dihydrochloride was successfully formulated by direct compression method the tablets were evaluated for Pre-compression parameters. As a result, this introduced the good flow and compressibility property of the bulk powder. Post compression parameters such as Physical parameter and chemical parameter was performed on punched tablets and found all the parameters within limit specified by Indian Pharmacopoeia. Keywords: Levocetirizine dihydrochloride, Direct compression, superdisintegrant, INVITRO dissolution, anti allergic tablet, Formulations.

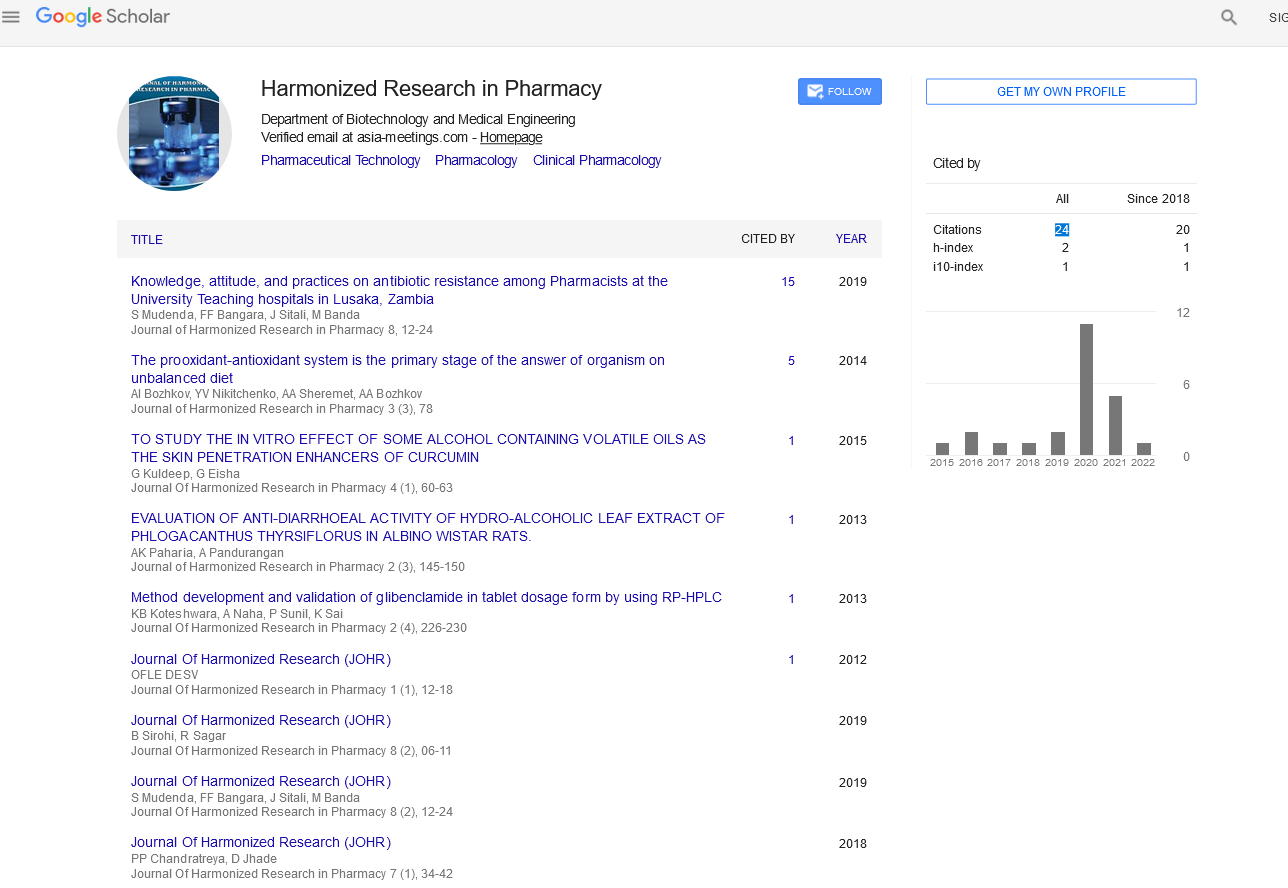
Google Scholar citation report
Citations : 147
Journal of Harmonized Research in Pharmacy received 147 citations as per google scholar report