MESOPOROUS SILICA SOLID DISPERSION CARRIER SYSTEMFOR SCANTY WATER SOLUBLE DRUG TO ESTABLISH CLINICAL DOSAGE FORMS
Abstract
Author(s): P Ambedkar* and Dr.E Harikrishna
Mesoporous MCM-41 is one of the mesoporous materials, used now-a-days as drug carrier. Itraconazole (ICZ) is a highly hydrophobic, BCS System class II drug.ICZ on oral administration results in poor bioavailability and inters individual variations in plasma drug concentrations due to poor aqueous solubility. Proximate target of the present study is to ameliorate the dissolution and bioavailability of Itraconazole by formulating the solid dispersions using mesoporous material as a carrier (MCM-41). Itraconazolesolid dispersions were prepared by solvent evaporation method polyethylene glycol-4000, 6000, polaxomer-407, HPMC, Gelucire 50/13, Gelucire 44/14, and mesoporous silica (MCM-41), among all the carriers mesoporous silica has delineated good results and exhibited dissolution enhancement confronts with the Itraconazole plain drug and marketed product. Stability was performed according to the ICH guidelines, accelerated, intermediate, and long term in stability results clearly demonstrated that mesoporous silica solid dispersions were stable. Key Words: Itraconazole, mesoporous silica, solubility, solid dispersions and gelucire

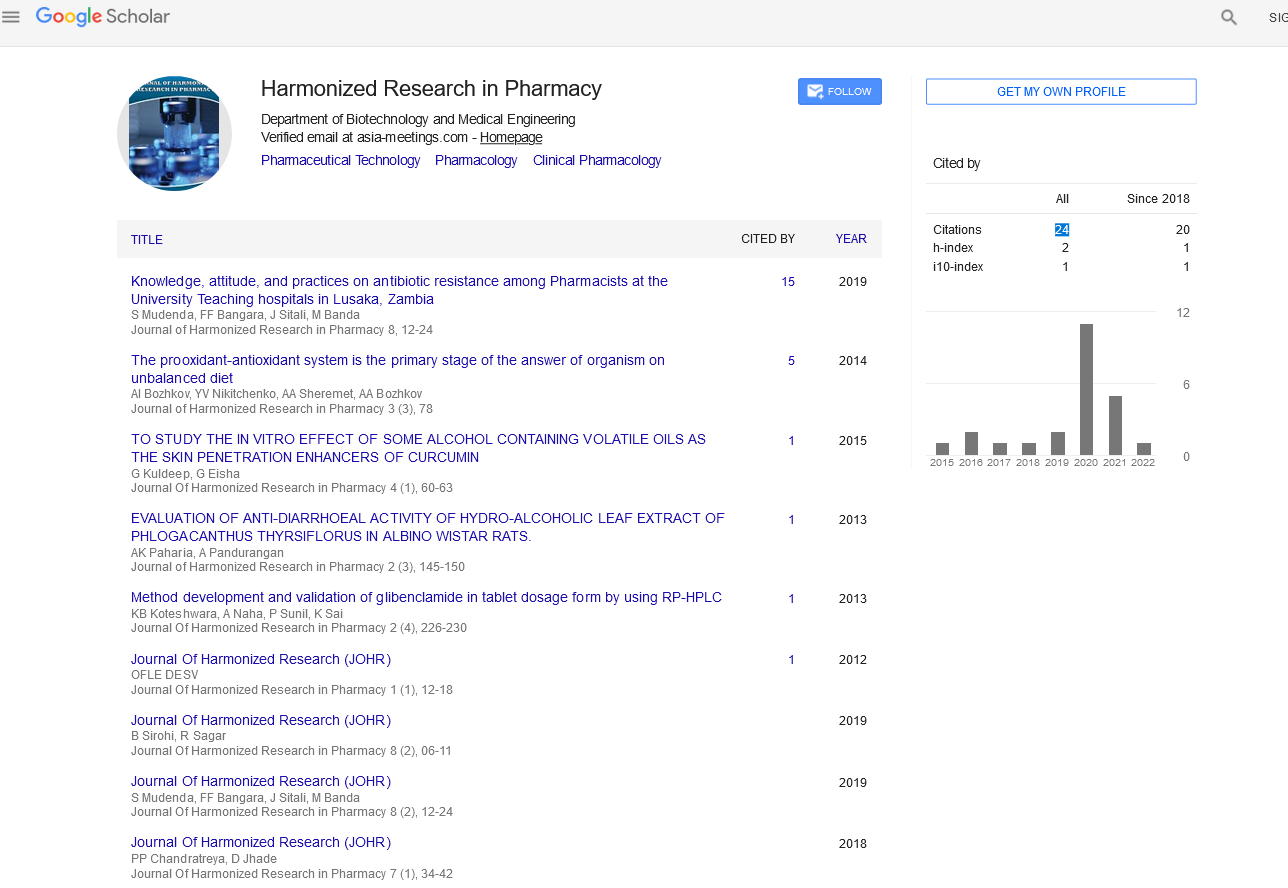
Google Scholar citation report
Citations : 147
Journal of Harmonized Research in Pharmacy received 147 citations as per google scholar report